Clinical Trials play a pivotal role in drug discovery, requiring seamless coordination among various stakeholders. Effective communication with committees, departments, sponsors, CRPs, and external resources is crucial throughout this process. Much like classifying workers as full-time, part-time, contractors, seasonal hires, and interns, clinical studies involve several key roles:

Now, let’s delve deeper into CRP onboarding, encompassing the following essential categories (though not exhaustive, they offer a representative sample, as each clinical study may have unique requisites):
Like any new position, appropriate training is required for CRPs to be successfull in this role. Typically, training falls into these buckets
General Training: CRPs require training in Good Clinical Practices (GCP), HIPAA (patient privacy), and informed consent procedures. For instance, completion of the Penn Clinical Research Onboarding Program within two weeks of hire or assignment via Workday Learning is mandatory for all CR staff.
Institutional Training: This includes training on financial conflict of interest.
Study-specific Training: CRPs must acquaint themselves with key departments supporting research, such as pharmacists and radiologists.
Informed consent is required document to be completed, usually this is a form accompanied by learning program.
Here is an comprehensive role based checklist that The Medical University of South Carolina has put together for CRP Onboarding
CRPs need access to various tools, including collaboration platforms, email, and systems like clinicaltrials.gov, Clinical Research Management, Research and Academic Systems, etc. ITSM processes, familiar to Jira users, often handle these access provisions.
By now, you may realize how valuable Jira Service Management and Jira Work Management can support Onboarding Clinical Research Professional workflows. Currently every medical university and research facilities have their checklists and templates, imagine we have centralized flows and administrators can customize flows to their individual needs.
Tailored workflows can streamline CRP onboarding, ensuring efficiency and clarity.

Flexibility in task management enables adaptation to various CRP roles.
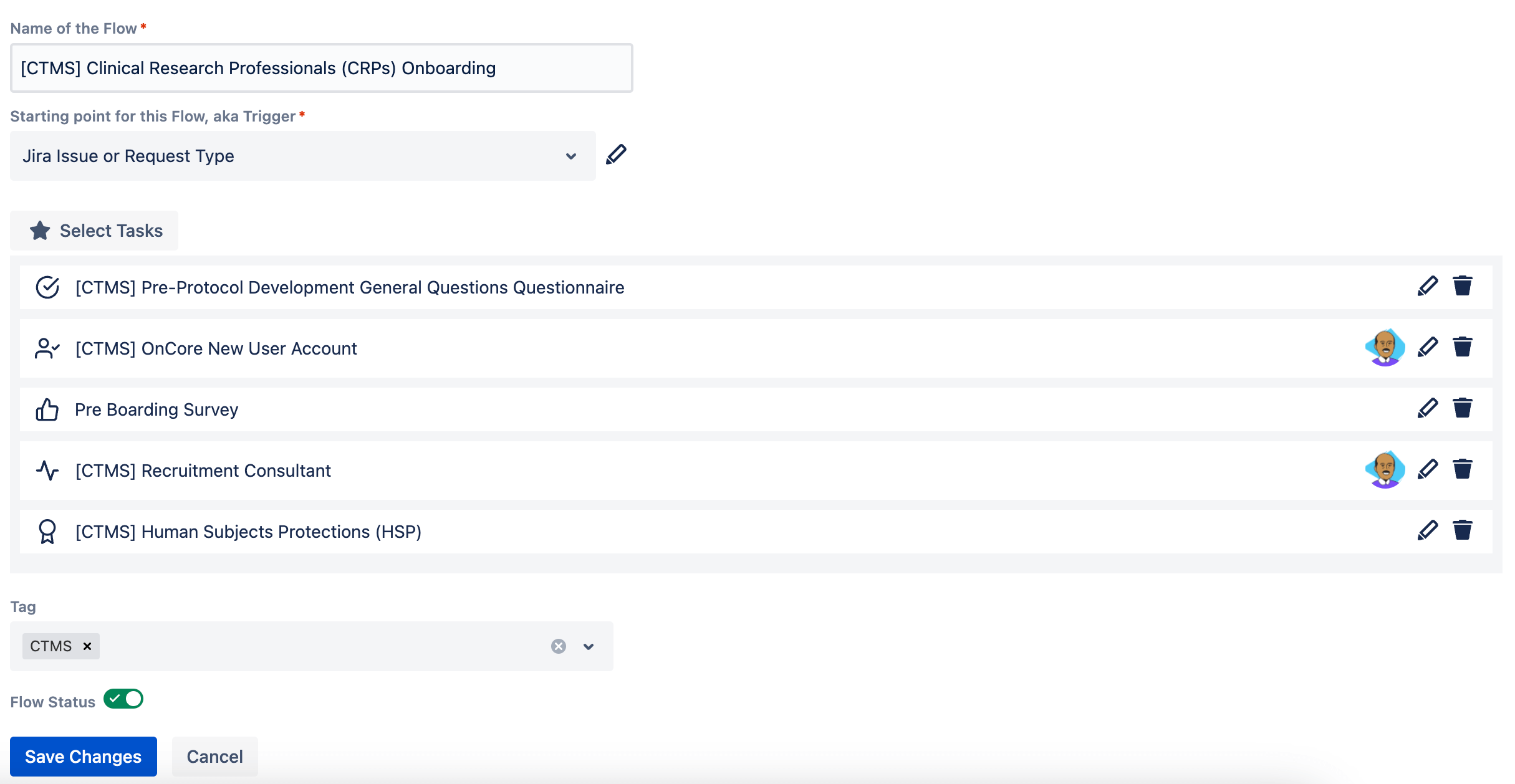
While project management, collaboration and communication provides immense value, automating repetitive tasks provide instant ROI. Having all the access provisioning automated removes IT friction and enables faster resolution and turn around times.
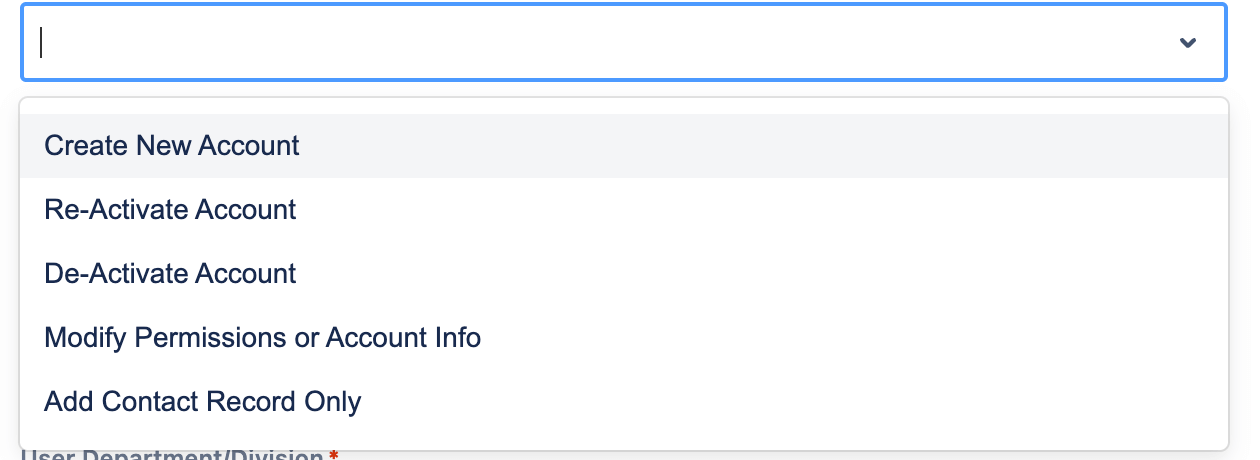
In this concise overview, we’ve touched on these critical aspects. Feel free to ask questions or provide feedback. I’d love to see more CRP onboarding utilizing JSM and take advantage of the features.
Duke Office of Clinical Research
Penn Clinical Research (CR) Onboarding Program
The Medical University of South Carolina
The University of Arizona Health Sciences – CRP Onboarding
Stanford Clinical Research Personnel Onboarding Checklist
Oregon Health and Science University
If these usecase are relevant for you, we request you to take a look at OnRamp. OnRamp is a native forge application exclusively focused on streamlining HR workflows for employee onboarding and offboarding. We have templates for role based CRP Onboarding.
Related Blogs
